Tin Complete Electron Configuration . 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony. This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. ← electronic configurations of elements. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. Full electron configuration of tin: 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. The complete electron configuration of tin is:
from www.alamy.com
It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. ← electronic configurations of elements. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. Indium ← tin → antimony. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the.
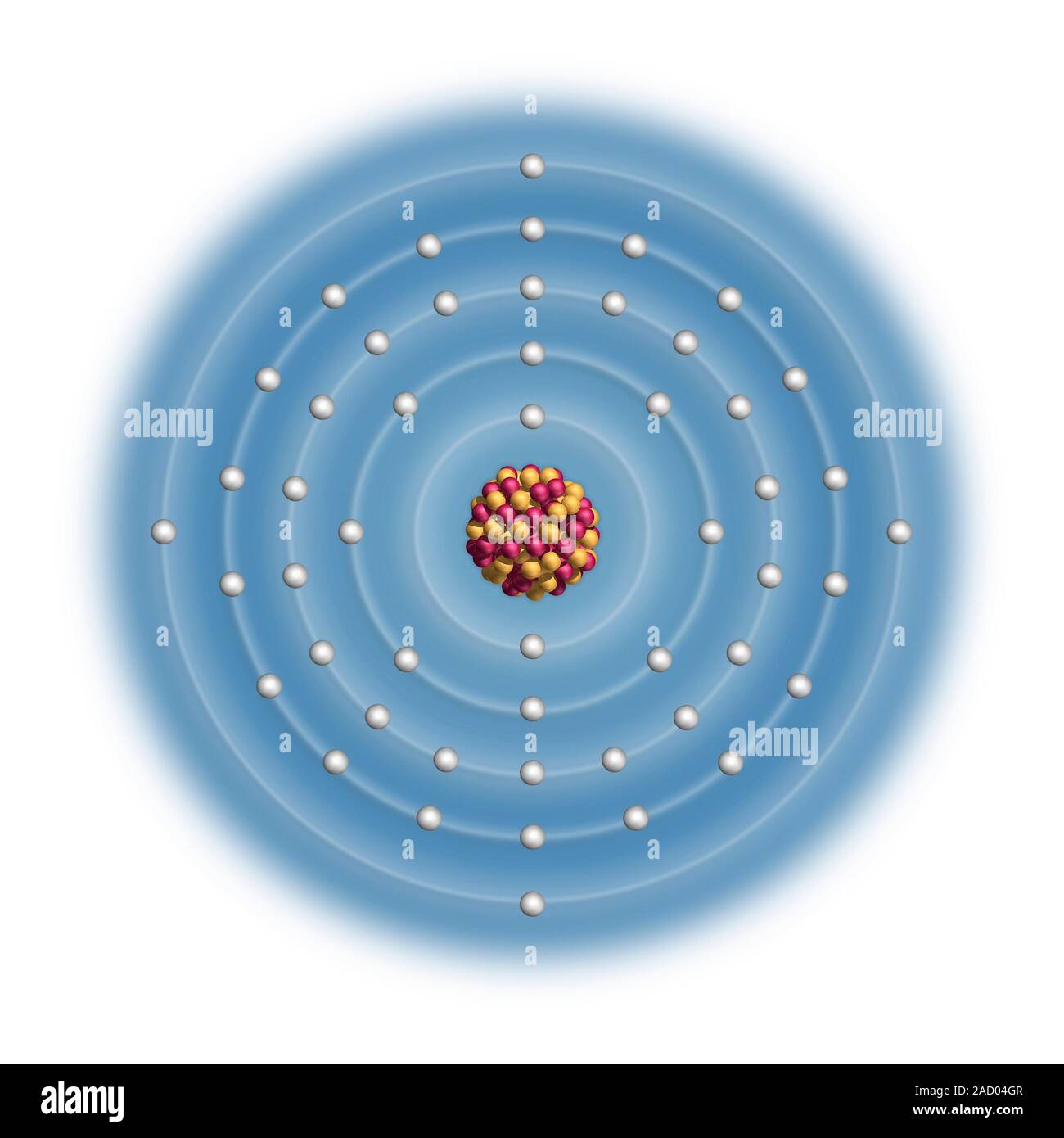
Tin (Sn). Diagram of the nuclear composition and electron configuration
Tin Complete Electron Configuration The complete electron configuration of tin is: Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. Full electron configuration of tin: ← electronic configurations of elements. Indium ← tin → antimony. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. The complete electron configuration of tin is:
From www.alamy.com
Tin atom Stock Vector Images Alamy Tin Complete Electron Configuration 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. Sn (tin) is an element with position number 50 in the. Indium ← tin → antimony. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has.. Tin Complete Electron Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Tin Complete Electron Configuration Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. Indium ← tin → antimony. This electron configuration shows that the tin ion(sn 4+) has four shells and the last. Tin Complete Electron Configuration.
From www.youtube.com
Full and Abbreviated Electron Configuration of Tellurium Te YouTube Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the. The complete electron configuration of tin is: This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. 119 rows electron configuration chart of all elements. Tin Complete Electron Configuration.
From www.animalia-life.club
Electron Configuration For Titanium Tin Complete Electron Configuration 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. To write the configuration. Tin Complete Electron Configuration.
From www.numerade.com
SOLVED Write the complete electron configuration for the zinc atom Tin Complete Electron Configuration To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. ← electronic configurations of elements. Indium ← tin → antimony. Full electron configuration of tin: Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Tin Complete Electron Configuration.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin Complete Electron Configuration To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the. ← electronic configurations of elements. Indium ← tin. Tin Complete Electron Configuration.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The complete electron configuration of tin is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. ← electronic configurations of elements. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. 119 rows electron configuration chart of all. Tin Complete Electron Configuration.
From es.lambdageeks.com
Configuración de electrones de estaño (explicada para principiantes) Tin Complete Electron Configuration 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. Sn (tin) is an element with position number 50 in the. ← electronic configurations of elements. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The complete electron configuration of tin is: It shows. Tin Complete Electron Configuration.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Complete Electron Configuration 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. The complete electron configuration of tin is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Full electron configuration of tin: Sn (tin) is an element with position number 50 in the. Indium ←. Tin Complete Electron Configuration.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration. Tin Complete Electron Configuration.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Tin Complete Electron Configuration This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. The complete electron configuration of tin is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2.. Tin Complete Electron Configuration.
From mavink.com
Ground State Electron Configuration Chart Tin Complete Electron Configuration Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. ← electronic configurations of elements.. Tin Complete Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that. Tin Complete Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Complete Electron Configuration Indium ← tin → antimony. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. 119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron. Tin Complete Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Complete Electron Configuration Full electron configuration of tin: Sn (tin) is an element with position number 50 in the. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. The complete electron configuration of. Tin Complete Electron Configuration.
From periodictableguide.com
Tin (Sn) Periodic Table (Element Information & More) Tin Complete Electron Configuration ← electronic configurations of elements. This electron configuration shows that the tin ion(sn 4+) has four shells and the last shell has eighteen electrons and it achieves a. Indium ← tin → antimony. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d. Tin Complete Electron Configuration.
From philschatz.com
Electronic Structure of Atoms (Electron Configurations) · Chemistry Tin Complete Electron Configuration It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Sn (tin) is an element with position number 50 in the. ← electronic configurations of elements. To write the configuration for the tin (sn) and the tin ions, first. Tin Complete Electron Configuration.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony. To write the configuration for the tin (sn) and the tin ions, first we need to write the electron. Sn (tin) is an element with position number 50 in the. Full electron configuration of tin: It shows a chemical similarity to both of its. Tin Complete Electron Configuration.